Describe How to Use Steel Wool to Remove Copper Ions
This solution will need at least 48 hours to form and is needed for the demonstration. B Copper compounds such as copperll sulfate are toxic.
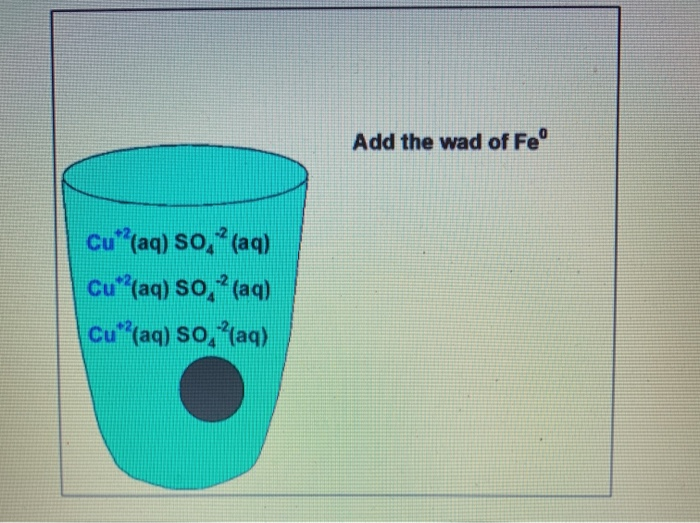
Procedure 5 Https Youtu Be Kmhd8bmeflo Fill A Chegg Com
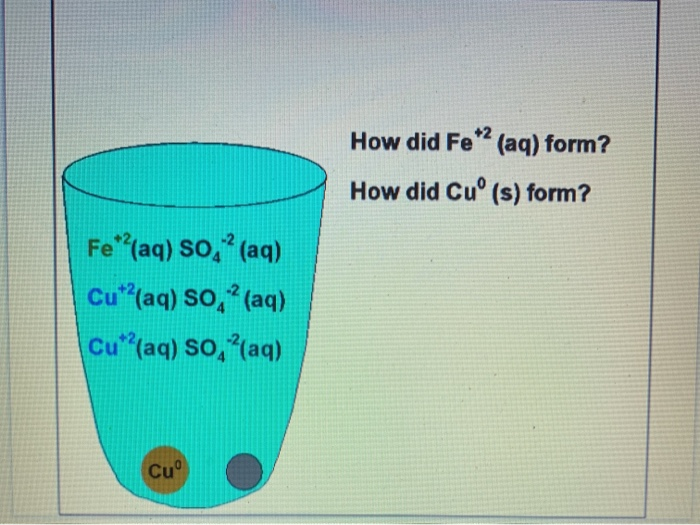
Fe s CuSO 4 aq - Cu s FeSO 4 aq the copper would form a precipitate and could be filtered from the solution FeSO 4 aq is non-toxic and actually used as a nutritional supplement.
. Actually it is called a Galvanic Reaction or Galvanic Corrosion. Be sure not to remove any copper from the beaker. C are the best.
Describe how to use steel wool which is made mostly of iron to remove the Cu2 ions from an aqueous solution of copperll sulfate. The fuzzy silver coating in Figure 3 of this section is impure silver. Put the object in a bucket of kitty litter then put the bucket on an oscillating object like an orbital sander to shake up the kitty litter and remove debris from the object by abrading the rust.
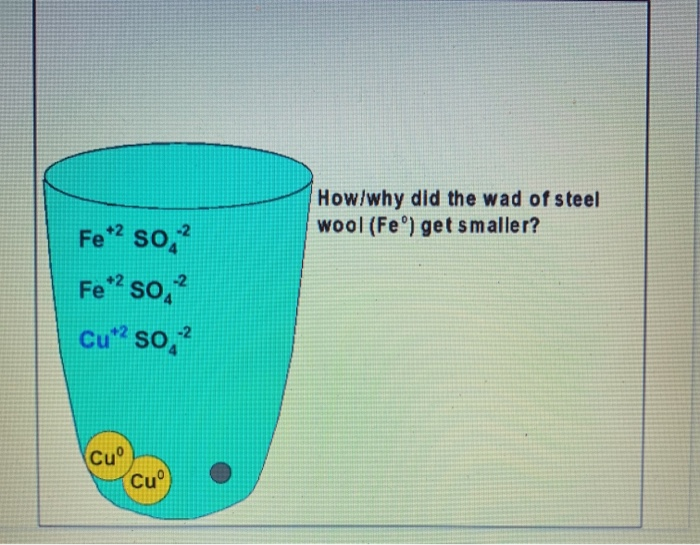
Due to the fact that the iron in the steel wool chemically reacts with the aqueous solution we can conclude that during the reaction the iron displaces the copper. B Copper compounds such as copperll sulfate are toxic. Use scissors to cut the 0000 grade steel wool from the bundle and then measure the mass to get 20 grams.
Describe how to use steel wool which is made mostly of iron to remove the Cu 2 ions from an aqueous solution of copperII sulfate. After reaction chemical equation Fe CuSo4 FeSo4 Cu steel wool copper sulfate Iron ii sulfate copper Florence. Soak some steel wool in white vinegar for a few hours or a few days the longer it steeps the darker the aged effect will be.
Describe how to use steel wool which is made mostly of iron to remove the Cu2 ions from an aqueous solution of copperll sulfate. Penguins vs avalanche 2022. Over time this can destroy the integrity of copper piping.
Describe how to use steel wool which is made mostly of iron to remove the Cu2 ions from an aqueous solution of copperll sulfate. Use 0000 steel wool shown here so it breaks down even faster in the vinegar. Disposal of copper sulfate This tiny penis has not yet been rated.
Describe how to use steel wool which is made mostly of iron to remove the Cu2 ions from an aqueous solution of copperll sulfate. Fill the jar with the steel wool and vinegar the measurements dont have to be precise. Copper compounds such as copper II sulfate are toxic.
Carefully break up any clumps of copper with a spatula if drying proves difficult. No acid is usedIn the subtractive process for making PC boards one of two etchant salt solutions will be used to remove copper from where traces are not desiredferric chlorideammonium. After Reaction A chemical property is the ability of a substance to change or react and.
In this case the copper would be. Before disposal these compounds must be treated to reduce their toxicity. Steel wool is available in several different strand thicknesses start with the largest thickness and work your way down to the fine one.
Before disposal these compounds must be treated to reduce their toxicity. Describe how to use steel wool which is made mostly of iron to remove the Cu 2 ions from an aqueous solution of copper II sulphate. Before disposal these compounds must be treated to reduce their toxicity.
For each run calculate the moles of iron used and the moles of copper formed. Describe how to use steel wool which is made mostly of iron to remove the Cu2 ions from an aqueous solution of copperll sulfate. When copper is displacedwe can then remove the separate the iron sulfate solution from the solid copper.
Describe how to use steel wool which is made mostly of iron to remove the Cu2 ions from an aqueous solution of copperll sulfate. Chemistry II - Concentrations. When the copper is dry carefully dry the outside of the beaker and reweigh to find the mass of copper formed.
Steps 2 and 3 describe how to make iron II acetate from white vinegar and steel wool. B Copper compounds such as copperll sulfate are toxic. Before disposal these compounds must be treated to reduce their toxicity.
Before disposal these compounds must be treated to reduce their toxicity. Steel touching copper water pipes can lead to electrolysis. B Copper compounds such as copperll sulfate are toxic.
Describe how to use steel wool to remove the Cu 2 ions from an aqueous solution of copper II sulfate. Before disposal these compounds must be treated to reduce their toxicity. To remove the tarnish from silverware soak it in a hot solution of baking soda in an aluminum pan.
B Copper compounds such as copperll sulfate are toxic. B Copper compounds such as copperll sulfate are toxic. Make iron II acetate 1 -2 days prior to using the demonstration.
Before disposal these compounds must be treated to reduce their toxicity. Disposal of copper sulfate.

Simple Chemical Reaction Experiment Steel Wool And Vinegar Reaction

Copper Ii Sulphate And Steel Wool By Florence Parweez

Chapter 8 Stoichiometry And Limiting Reagent Copper Chloride Lab

Steel Wool And Vinegar Wood Aging Ebonizing Weathering A Controlled Experiment Instructables

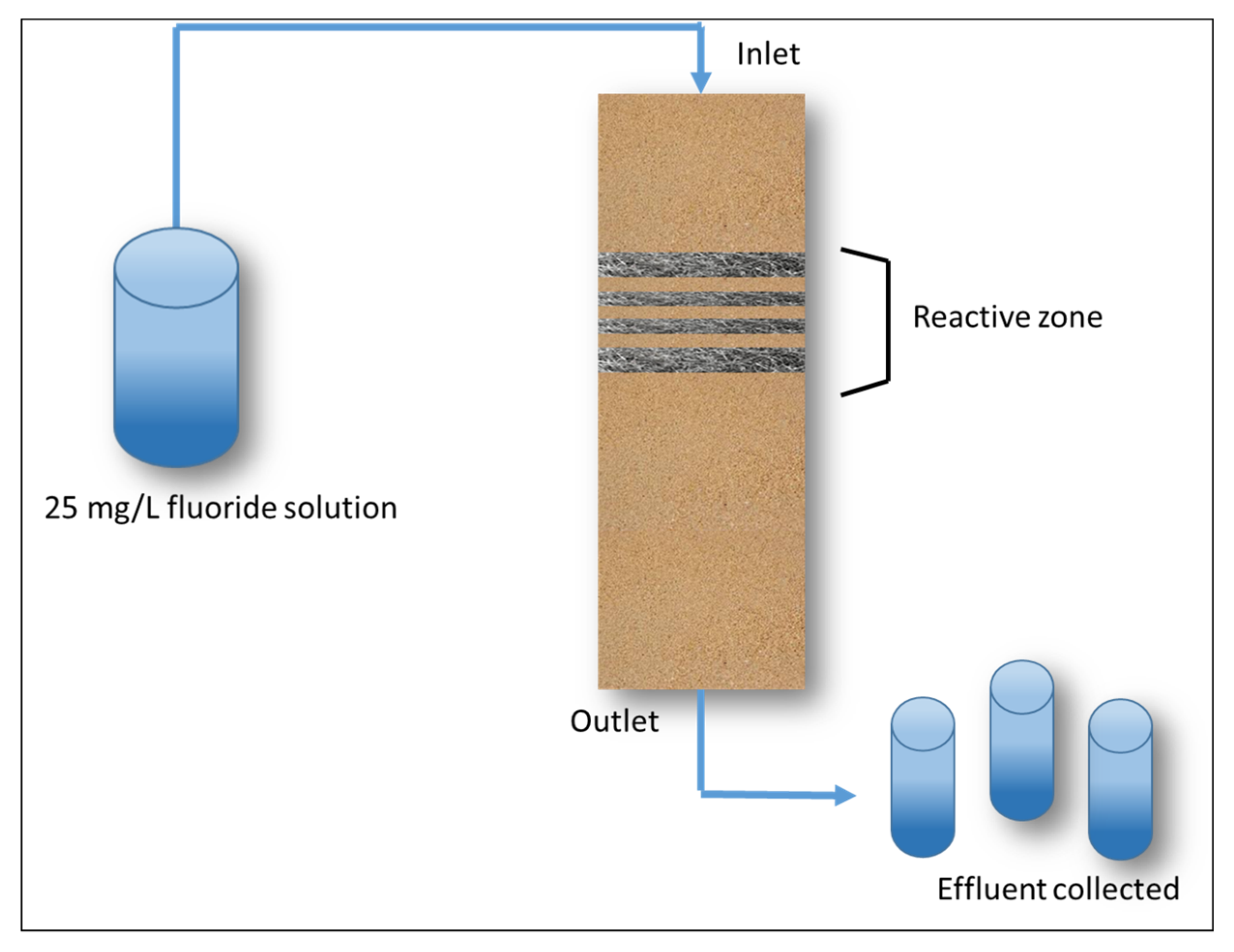
Processes Free Full Text Steel Wool For Water Treatment Intrinsic Reactivity And Defluoridation Efficiency Html

Photographs Of Six Steel Wool Specimens Found On The Market In Download Scientific Diagram

Simple Chemical Reaction Experiment Steel Wool And Vinegar Reaction

How To Make Steel Wool And Vinegar Stain Youtube

Procedure 5 Https Youtu Be Kmhd8bmeflo Fill A Chegg Com
Procedure 5 Https Youtu Be Kmhd8bmeflo Fill A Chegg Com
Procedure 5 Https Youtu Be Kmhd8bmeflo Fill A Chegg Com
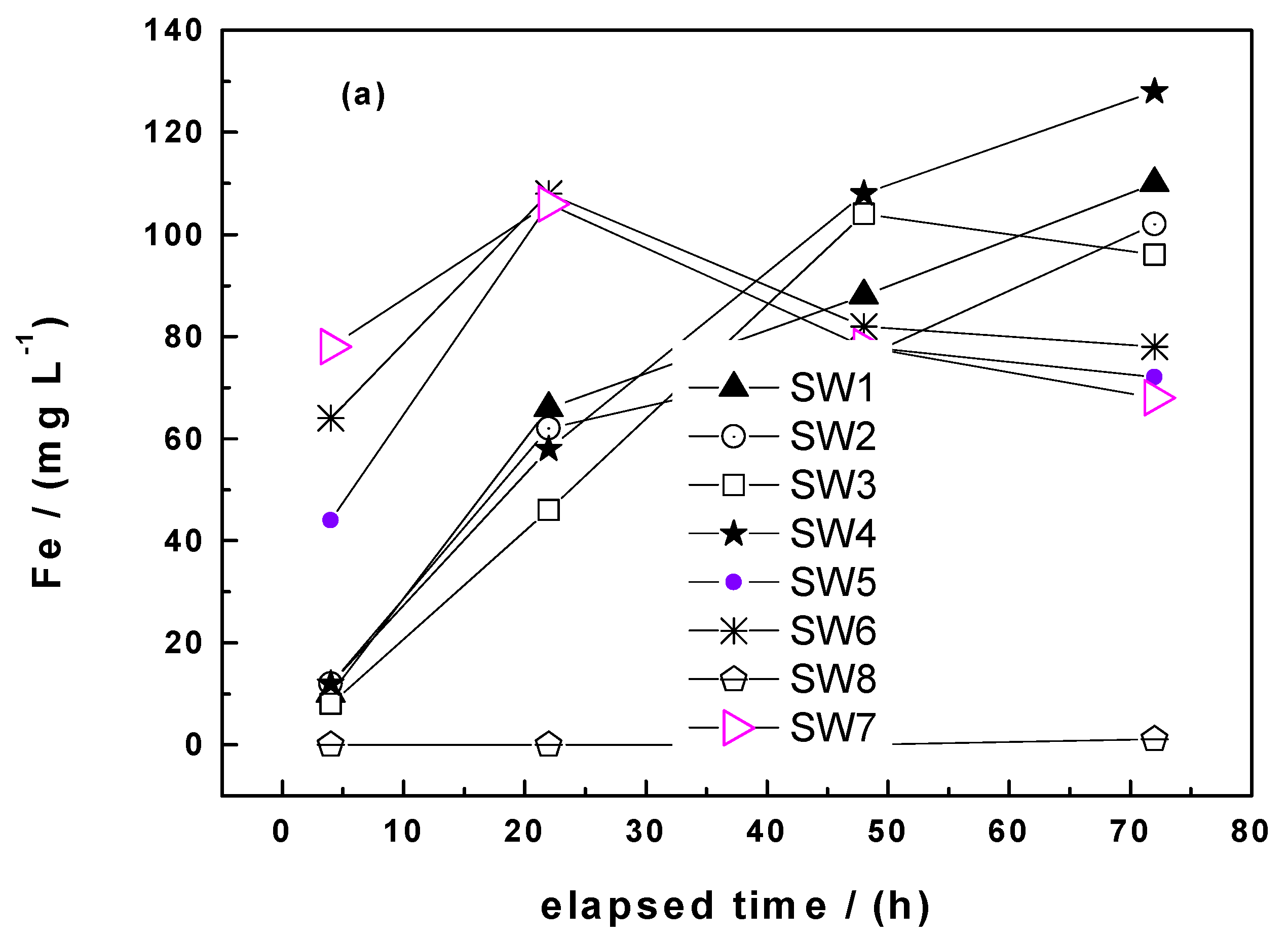
Processes Free Full Text Steel Wool For Water Treatment Intrinsic Reactivity And Defluoridation Efficiency Html

Placing Steel Wool Into A Copper Ii Sulfate Cuso4 Solution Youtube
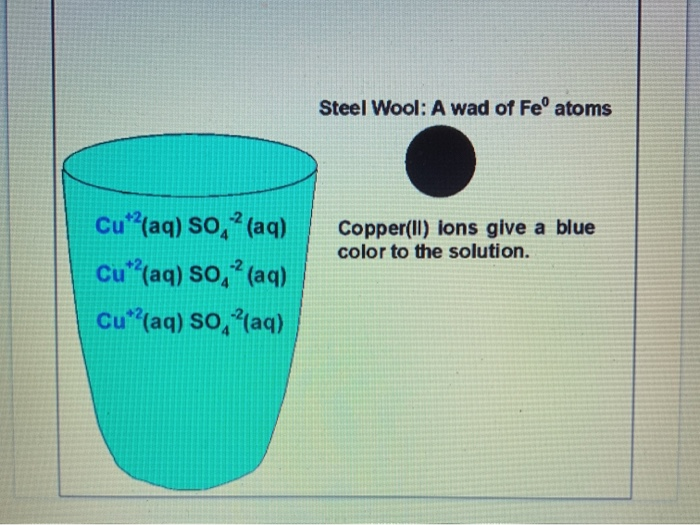
Procedure 5 Https Youtu Be Kmhd8bmeflo Fill A Chegg Com

Photographs Of Six Steel Wool Specimens Found On The Market In Download Scientific Diagram

Copper Ii Sulphate And Steel Wool By Florence Parweez

Steel Wool And Vinegar Wood Aging Ebonizing Weathering A Controlled Experiment Instructables
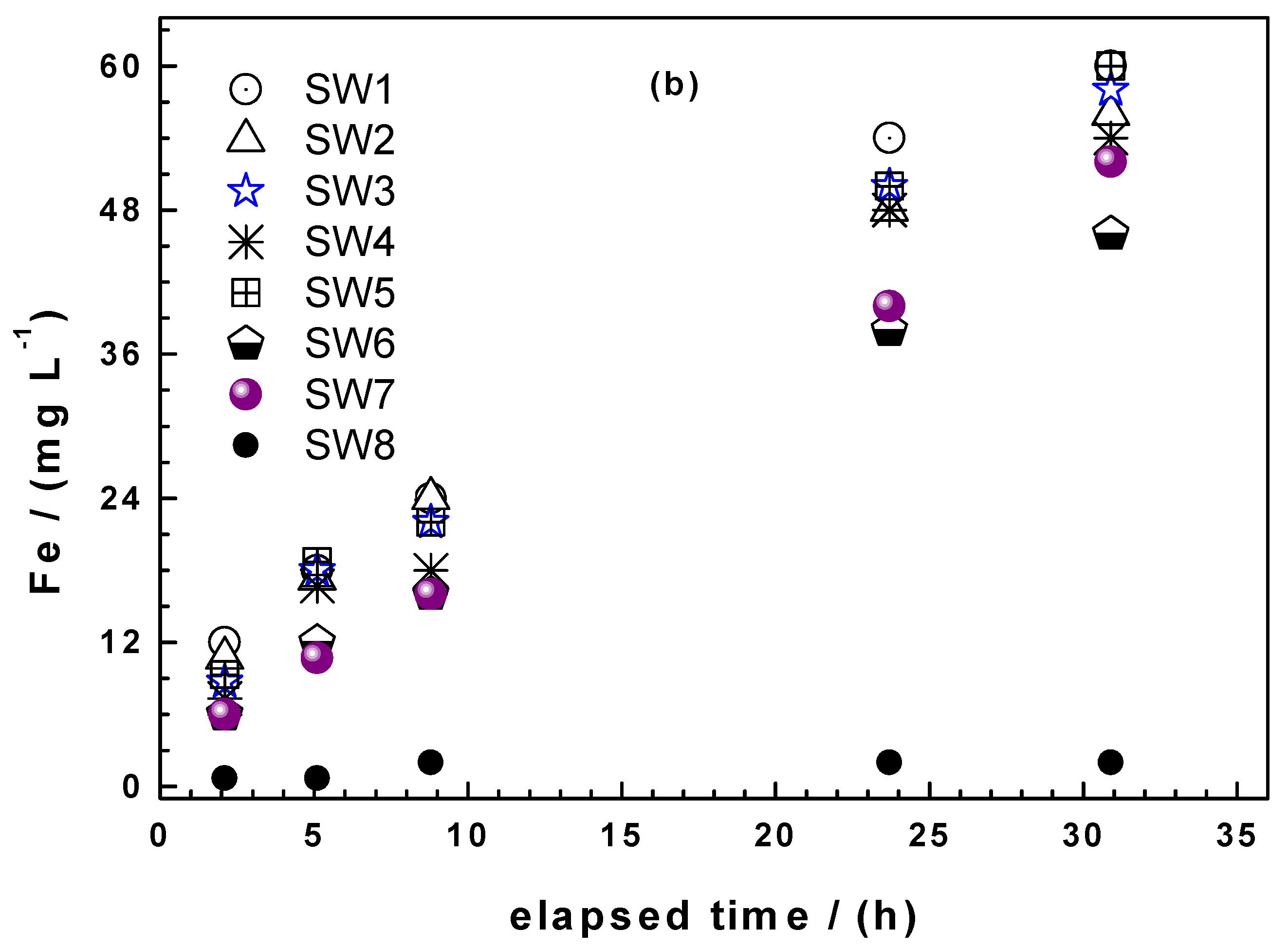
Processes Free Full Text Steel Wool For Water Treatment Intrinsic Reactivity And Defluoridation Efficiency Html
Comments
Post a Comment